 To date only Singapore has developed standards for cell cultured meat. Israel is due to follow, given government support and advanced technology from universities facilitating at least three major developers, all heavily supported by venture capital and major protein producers.
To date only Singapore has developed standards for cell cultured meat. Israel is due to follow, given government support and advanced technology from universities facilitating at least three major developers, all heavily supported by venture capital and major protein producers.
It is not expected that the United States will soon release a comprehensive set of standards and regulations, given the split jurisdiction between the USDA on production and FDA on technology. It would appear that both U.S. agencies are approaching cell cultured meat from the their narrow perspectives of conventional food production and safety. What is needed is a novel and coordinated approach through a central food safety agency yet to be established.
In the E.U. cell based meat will fall under the Novel Food Regulation issued in 2018. This document has yet to be approved by all member states and despite technical progress in the Netherlands, commercialization will be years in the future.
The Center for Rural-Marketing Strategies, a quasi-government body in Japan, is considering regulations, but again it is noted that Japan moves slowly on food-related innovations.
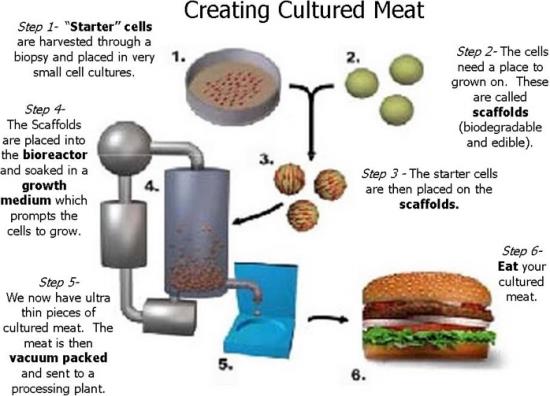
China is reviewing regulations for cell-cultured meat. The Nation has the technical capability to establish standards and issue regulations within a year. This would require a directive from the highest levels of the central government and only after evaluation of the economic, political, and social desirability of an alternative source of protein.
As technology is advancing it may be possible within a few years to achieve cost parity with conventional meat. Accordingly the regulatory framework is urgently required to allow developers to plan production facilities and procedures that will be compliant with standards A regulatory framework will be necessary for investors to commit funds to a new industry that will require considerable fixed and working capital. A further complication will be the inevitable lack of harmonization in standards among North America, the E.U.-U.K. and Asia that will result in islands of production and limited trade.
Apart from the regulatory considerations, there are profound questions as to consumer acceptance. Vegetarians will in all probability reject cell cultured products. There will be intense competition from both plant-based and natural meat. Consumers who regard sustainability and welfare as important attributes will probably not represent a sufficiently large base on which to establish an extensive industry capable of becoming a significant source of protein.